2018.10.13; Healthy mice from same-sex parents have their own pups
Published by DB,
Healthy mice from same-sex parents have their own pups
A female mouse, born to two mothers, tends to her own pups.Credit: Leyun Wang
For the first time, researchers have used the DNA from two mouse mothers to create healthy pups, some of which matured and had their own offspring. The scientists also produced baby mice using the combined genetic material from two fathers, although those pups only lived for a couple of days.
The method the team used to create the pups, described in a study1 published on 11 October in Cell Stem Cell, reveals important genetic factors necessary for the development of healthy embryos. But scientists are sceptical that the technique could ever be applied to people.
Some animals, such as certain species of birds, fish and lizards, can reproduce using only one sex or an individual. Mammals, however, need members of the opposite sex to create the next generation.
Scientists think this is because of genetic imprints, small chemical tags that attach to DNA and turn off a gene. They’ve found roughly 100 such tags, many of which are found on genes affecting an embryo’s growth.
Many genes that are tagged in one sex remain untagged in the opposite sex. Combining two of the same tagged genes in an embryo — which would happen with parents of the same sex — leads to its death.
Deleting tags
Attempting to overcome this barrier, study author Qi Zhou, a developmental biologist at the Chinese Academy of Sciences in Beijing, and his team used lab-grown embryonic stem cells from either a sperm or an egg. These cells have only one set of chromosomes and, like most cells, contain genetic regions that can produce the chemical tags.
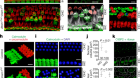
In a process of trial and error, and on the basis of results from previous studies2, the researchers deleted these genetic regions in batches, searching for groups that could be removed without stopping the production of a healthy embryo. The team then combined a stem cell from a female mouse with the egg from another female to create pups from two mothers. They also took a stem cell from a male and injected it, along with another male’s sperm, into an egg without a nucleus to create offspring from two fathers.
After deleting three genetic regions, the scientists managed to produce 29 living mice from two females, 7 of which went on to have their own pups. The team needed to delete 7 regions to produce 12 pups from two male parents — but those baby mice lasted only 2 days after succumbing to problems including trouble breathing and extra fluid in their tissues.
These results revealed some of the most important genetic regions that prevent mammals from reproducing without two individuals of the opposite sex, says Zhou. It also showed “a new and clear way to produce offspring between same-sex mammals”.
Risky business
Scientists are sceptical that this technique could ever be applied to humans, however. “Most, if not all, of the embryos that they developed were still abnormal and could not survive,” says Jacob Hanna, a molecular geneticist at the Weizmann Institute of Science in Rehovot, Israel. The authors only had a 14% success rate with embryos from the two mothers and a 2.5% rate with the two fathers.
“I think it’s almost impossible that this would be allowed for clinical application,” Hanna says.
“When you reproduce, you really want every factor possible to have a good outcome,” says Allan Spradling, a reproductive biologist at the Carnegie Institution for Science in Baltimore, Maryland. But nothing indicates how normal these mice are, such as how susceptible they might be to diseases, he adds.
“I don’t think it’s going to lead to people genetically having two mothers or two fathers as a routine thing,” says Spradling. “We don’t understand it well enough, and it might be too risky to take it that far.”
Latest on:
Developmental biology
Epigenetics
Stem cells
Sign up for the daily Nature Briefing email newsletter
Stay up to date with what matters in science and why, handpicked from Nature and other publications worldwide.
Li, Z. et al. Cell Research 26, 135-138 (2016).